Most people call them COLAs or FLAs (federal label approvals) or “label approvals.” But those terms leave out the not so minor B — as it appears in the word “bottle,” highlighted above. TTB’s pre-market approval system extends to bottles, and it is starting to seem like many people forgot about this or never knew. TTB’s bottle review probably does not cover run of the mill bottles. It is meant to cover “distinctive bottles.” The COLA form mentions that you must complete item 18.c. “if you intend to bottle distilled spirits in a distinctive container.” It’s not so easy to know what is and isn’t distinctive. The regulations use the term “distinctive” many times, and even explain the requirements for distinctive bottles, but they don’t explain when bottles are and aren’t. It is good to know that Jim Beam Brands, at least, still knows how to do this right, and can serve as a good reminder to others. The above Stillhouse Decanter certainly appears to be — on the distinctive side — for a bottle. And alas, the corresponding approval is here; item 18.c. seems to be duly completed to verify that the bottle is both distinctive and approved. Beam’s press release puts things in perspective and explains that even Jim Beam does not...
Continue Reading Leave a Commentalcohol beverages generally
Moonshine

Moonshine. A word that typically conjures up thoughts of illicit high-octane liquor, clandestine stills, mason jars, potential blindness and bearded mountain men with colorful nicknames. Producing moonshine without a license is still illegal in the United States, but a large and growing number of licensed distilleries are now producing their own interpretations of moonshine. And despite moonshine’s negative associations from the past, TTB seems to have no issue allowing the word to appear on distilled spirits labels, as evidenced by the scores of moonshine labels approved so far. There is also an upsurge in approvals for moonshine’s cousins, such as white dog, white whiskey and white lightning. As far as we know, there are no specific TTB requirements to label a product “moonshine.” Apparently, moonshine can be a whiskey, a specialty product with flavors of apple or blackberry (for example), a high poof neutral spirit distilled from apples, peach brandy and even tequila. Although it appears that you can call just about any distilled spirits product “moonshine,” we think it is unlikely that TTB would allow the word on beer or wine labels anytime soon.
Continue Reading Leave a CommentProof: Booze Makes the World More Colorful

For quite some time, I have noticed that alcohol beverage packaging tends to be prettier than lots of other packaging. Now, perhaps, I am on the verge of proving this hunch, though the manner of proof, in the form of a BuzzFeed article, may be a bit light on evidence. The article shows the “34 Coolest Food Packaging Designs Of 2012.” Of this sampling, fully 20 are beverages. Of those, no less than 13 (more than a third) are alcohol beverages. Not bad, considering all the other categories represented, such as chocolate, cheese, jam, pasta, and bread. Within the alcohol beverage category, I think the Slamsey’s Gin (as above) and Dancing Pines Bourbon bottles look good. I did not notice US approvals for those two, or most of the others on the list, so far. So this may be a harbinger that there is plenty of interesting work to look forward to in 2013. Of the products listed, Kraken Spiced Rum is the most familiar, and the US approval is here.
Continue Reading Leave a CommentTags: design
FDA Not Ready For FSMA Renewals
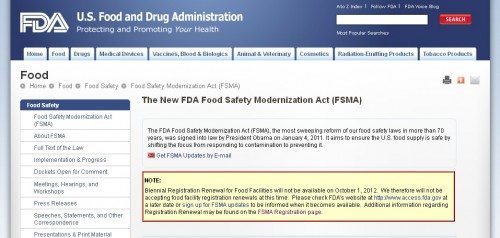
Under the new and important Food Safety Modernization Act (FSMA), FDA was supposed to commence food facility re-registrations yesterday. This was mandated by section 102 of the FSMA law, enacted in 2011. This piece of the FSMA puzzle is not off to a propitious start. It was bad enough that there was not much guidance or clarity about how this would work, before the October 1, 2012 to December 31, 2012 re-registration period began. But it’s even worse that the brief window for required and biennial re-registration began yesterday — and yet there is still no means by which to accomplish what is required. The renewal website was briefly available last week, for a few hours, then it froze, then it disappeared, to be replaced with an oh so calm assurance that:
Biennial Registration Renewal for Food Facilities will not be available on October 1, 2012. We therefore will not be accepting food facility registration renewals at this time.
Natural Products Insider has explained:
Continue Reading Leave a CommentFDA is delaying the registration renewals that are mandated under a 2011 law after the Grocery Manufacturers Association and numerous other trade associations recently sought guidance in meeting the registration requirements. “It would be extremely inefficient and costly for companies to re-register shortly after October 1st based on the...
Tags: fda
Your New Friend, ARTAL
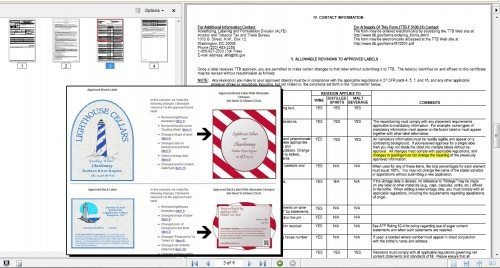
In early July TTB announced a massive and important change to the COLA system. TTB greatly expanded the “Allowable Revisions to Approved Labels” (hereinafter “ARTAL,” as on page 3 of the new 4-page COLA form). TTB began laying the groundwork for big “streamlining” changes in early 2012, as summarized here. Although some of the ideas seemed very modest as of then, the streamlining train clearly picked up momentum in the next few months. It seems entirely possible that some of the new changes could or should cut a very large percentage of the more than 10,000 labels submitted to TTB every month. Compared to a few years ago, it is quite amazing that the lighthouse label on the left (above) could change to something as different-looking as the striped label on the right — without any need for a new COLA. The TTB ID number on this label, for example, shows that TTB received at least 671 label applications on just one day in April 2012 — to say nothing about the labels submitted via paper. That should not happen anymore. Instead, applicants should get familiar with ARTAL. It can eliminate lots of waiting, expense, frustration, inconsistent determinations, TTB work and applicant work. In my view, the biggest changes to ARTAL are these....
Continue Reading Leave a CommentGluten Free Ruling

TTB announced a big policy change — about gluten free — just before the Memorial Day holiday weekend. For many years before the announcement, plenty of companies have tried to make “gluten free” claims, but we still didn’t see any approved TTB labels referring to “gluten free.” A few weeks back, we thought we had one, when we heard a lot of buzz about Omission beer as above. But alas, even the Omission label has had a big omission when it comes to this particular claim. All this is about to change in a big way, as result of this TTB Ruling, released late last week. As a result, we may begin to see various gluten free claims on TTB labels in the very near future. To show the earlier TTB policy, a fairly recent TTB rejection is here, and it may help explain why Widmer did not come out and say it louder or earlier. TTB’s caution may well have been justified; Brewbound has explained: “The release of Omission Beer comes just a few months after a study published in the Journal of Proteome Research found that eight commercial beers currently labeled as ‘gluten-free’ contained as much gluten as regular beer.” Brewbound further explained that there are plenty of other beers that seek...
Continue Reading Leave a CommentTags: policy, rejections, therapeutic


