For quite some time, I have noticed that alcohol beverage packaging tends to be prettier than lots of other packaging. Now, perhaps, I am on the verge of proving this hunch, though the manner of proof, in the form of a BuzzFeed article, may be a bit light on evidence.
The article shows the “34 Coolest Food Packaging Designs Of 2012.” Of this sampling, fully 20 are beverages. Of those, no less than 13 (more than a third) are alcohol beverages. Not bad, considering all the other categories represented, such as chocolate, cheese, jam, pasta, and bread.
Within the alcohol beverage category, I think the Slamsey’s Gin (as above) and Dancing Pines Bourbon bottles look good. I did not notice US approvals for those two, or most of the others on the list, so far. So this may be a harbinger that there is plenty of interesting work to look forward to in 2013. Of the products listed, Kraken Spiced Rum is the most familiar, and the US approval is here.
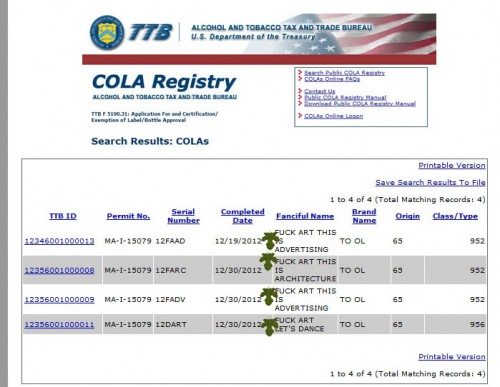
Shelton F's with Beer, Art, and Commercial Speech
Way back in mid-December of 2012 I would have considered this Shelton Brothers COLA to be, perhaps, an aberration. But upon checking it again, today, I see a few more COLAs with the same word — arguably in need of the fig leafs above.
It is hard to believe that the government did not see the word at issue. On the above-linked COLA it appears no less than three times. This may signal that, as social mores liberalize and budgets shrink, the government has bigger (or fewer) fish to fry. Clearly, it signals that Daniel Shelton does not mind pushing the envelope, or many. The Amherst College magazine unabashedly explains that, after graduating from Amherst, Shelton:
went to a prestigious law school … then clerked for a judge (on a tropical Pacific isle, of all places) and finally secured a position at a venerable firm in Washington, D.C. (but convinced Shea & Gardner that he needed to spend a year bumming around Africa before starting.) … “My Amherst education has not been wasted at all. I use it more in this business than I ever did in lawyering. I never was completely comfortable with the idea of being a lawyer, anyway.”
This creaky old regulation still prohibits any beer labeling that is “obscene or indecent.” At this rate, however, it is difficult or uncomfortable to imagine something that goes too far — or too far for Dan. Many thanks to Mark for showing me these labels.
Is Wine Vegan?
Not that I read the PETA stuff every day, but I could not resist when I stumbled on PETA’s article entitled, “Is Wine Vegan?” It makes the point that:
The majority of people are unaware that wine, although made from grapes, may have been made using animal-derived products. During the winemaking process, the liquid is filtered through substances called “fining agents.” This process is used to remove protein, yeast, cloudiness, “off” flavors and colorings, and other organic particles. Popular animal-derived fining agents used in the production of wine include blood and bone marrow, casein (milk protein), chitin (fiber from crustacean shells), egg albumen (derived from egg whites), fish oil, gelatin (protein from boiling animal parts), and isinglass (gelatin from fish bladder membranes). Thankfully, there are several common fining agents that are animal-friendly and used to make vegan wine. Carbon, bentonite clay, limestone, kaolin clay, plant casein, silica gel, and vegetable plaques are all suitable alternatives.
For those who would prefer not to torment an animal in the course of pouring a glass of wine, The Vegan Wine Guide already lists more than 400 wines. The Vegan Vine seems like a good example. As I flipped through a few of the 400, I was not surprised to see that few if any make direct claims that the wine qualifies as “vegan.” After all, TTB is not known for being footloose and fancy-free about various claims. Foursight Wines has said:
[we were] pleased that the TTB allowed us to state that our wines are suitable for vegetarian and vegan diets. Clos La Chance has begun marketing all vegan wines, but the TTB didn’t allow them to say that the wines didn’t use animal products (see the Wines & Vines article here). Frey is a noted vegan producer but their wines don’t list it on the label. So, unless anyone out there has a correction for me, I have yet to find another U.S. producer with a vegan and vegetarian statement on their wine labels.
If your tastes run in the other direction, you may prefer these libations replete with animal byproducts.
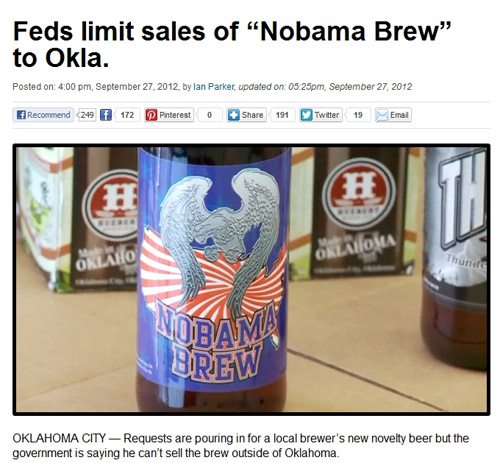
Nobama Beer
What is it about beer that encourages people to say things — they would never want to say on cheese or ketchup labels? In the latest skirmish, an Oklahoma brewer came out with Nobama Beer during the past few weeks.
It appears that TTB was not too fond of this brand name, at least at first. But then Huebert Brewing Company, their lawyer, and the local NBC affiliate went on the offensive, to push the label through, as shown in this video. I must admit, I did not expect to see a TV news story about the finer points of TTB Form 5100.31, Exemptions from Label Approval, or TTB’s renowned beer label reviewer (the one person that has reviewed and approved the label for just about every beer currently available in the US). The first video shows that TTB at first allowed the beer only within Oklahoma, but the above approval, and this later video, shows that TTB shortly thereafter felt compelled to allow it more widely.
The examples of envelope-pushing beer labels are probably too numerous to mention here. And they are certainly not limited to the Obama bashers, as in this example.
Chokin' Chicken Vodka
You read that right. It’s far from Chopin Vodka. It’s Chokin’ Vodka. Chokin’ Chicken Vodka to be more precise. This may signal that it’s time for the Wild Turkey and even the Rex Goliath to step aside and make way for another bird.
We are pleased to see that many fun, inventive labels keep going through. I am a little surprised that it was ok to say “Not intended To Grow Hair On A Goat’s Ass.” Chokin’ Chicken is bottled by Gatlinburg Barrelhouse LLC of Gatlinburg, Tennessee.
FDA Not Ready For FSMA Renewals
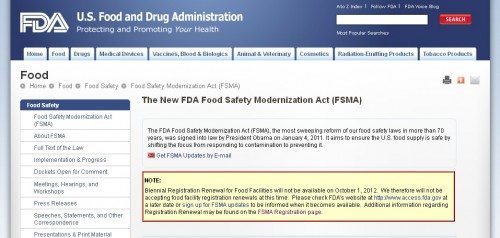
Under the new and important Food Safety Modernization Act (FSMA), FDA was supposed to commence food facility re-registrations yesterday. This was mandated by section 102 of the FSMA law, enacted in 2011.
This piece of the FSMA puzzle is not off to a propitious start. It was bad enough that there was not much guidance or clarity about how this would work, before the October 1, 2012 to December 31, 2012 re-registration period began. But it’s even worse that the brief window for required and biennial re-registration began yesterday — and yet there is still no means by which to accomplish what is required. The renewal website was briefly available last week, for a few hours, then it froze, then it disappeared, to be replaced with an oh so calm assurance that:
Biennial Registration Renewal for Food Facilities will not be available on October 1, 2012. We therefore will not be accepting food facility registration renewals at this time.
Natural Products Insider has explained:
FDA is delaying the registration renewals that are mandated under a 2011 law after the Grocery Manufacturers Association and numerous other trade associations recently sought guidance in meeting the registration requirements.
“It would be extremely inefficient and costly for companies to re-register shortly after October 1st based on the old procedures, only to find out later they have to do it all over again after FDA clarifies the new procedures in its guidance,” wrote Leon Bruner, senior vice president, science and regulatory affairs, and chief science officer of the Grocery Manufacturers Association, in a Sept. 21 letter to the Office of Management and Budget. “Thus, it will be difficult, if not impossible, for food facilities to effectively and efficiently meet FSMA’s registration renewal mandate without guidance from FDA.”
This leaves several hundred thousand food facilities around the world, plus their required agents, with a looming and ever closer deadline, but no means by which to comply with the law.
A few weeks earlier, in late summer, two groups sued FDA “for declaratory and injunctive relief regarding the failure by [FDA] to promulgate final regulations by mandatory deadlines contained in [FSMA].” The non-profit food groups said:
FDA has missed not one, not two, but seven critical deadlines, and counting, in failing to implement FSMA’s major food safety regulations. FDA has submitted several of these unlawfully delayed regulations to [OMB], where they are still awaiting approval. However, FDA has authority to promulgate the regulations without OMB approval.
Despite this bump in the road, here’s what food companies around the world can do, to avoid missing the renewal deadline. Make sure you have a reputable FDA food agent, if you are based outside the US. Make sure that agent has up-to-date information about your facility. You should be especially careful if your agent hides its true identity, or has vaguely (and confusingly, aggressively, and in many cases not so vaguely) pretended to be affiliated with FDA. Some of the agents charge as much as $900 in the first year. If your US importer, or a friend, has handled this for you in the past, it may be time to reconsider and at least make sure your agent is aware of the changed environment. The law can subject the US agent to substantial liability for the costs related to recalls and re-inspections.
For more information about agents and registration, go to www.food-agent.com. The site is affiliated with Lehrman Beverage Law, a law firm in the Washington, DC area that has been acting as US agent for hundreds of companies around the world, since the earliest days of the FDA agent requirement, almost 10 years ago. Unlike many other leading agents, food-agent works within traditional attorney ethics rules, has moderate fees, and tries to avoid confusing food companies about their identity or their relationship with FDA.
October 19, 2012, 9 pm Update: FDA has announced that “Biennial registration renewal for food facilities will begin at 12:01 a.m. on October 22, 2012. At that time, the system will be accepting food facility registration renewals.”